Product Categories
Calibration and Measurement for Ammonia Ion Selective Electrode in the Lab

The measurement of ammonia is vital in applications such as wastewater, where the wastewater treatment plants are required to provide a laboratory measurement of ammonia concentration. The ammonia gas-sensing ion selective electrode (ISE) is an EPA approved method of determining ammonia concentration for compliance reporting.
Ammonia Ion Selective Electrode Measurement Principle
An ammonia gas-sensing ISE contains two main structures - a pH electrode and a gas permeable membrane module. There is a unique ionic strength adjustor (ISA) that is used with the ammonia ISE which buffers the sample to a pH greater than 11. This causes the ammonia in the sample to become gaseous.
The pH electrode fits inside the membrane module and the sensing end of the electrode is immersed in internal fill solution. There is a permeable membrane that the ammonia gas will pass through which results in a pH shift within the internal fill solution. This pH shift is detected by the pH electrode and can be correlated to the concentration of ammonia in the sample based on the result of calibration.

Setting Up the Ammonia Ion Selective Electrode
When setting up the ammonia ISE, the soaking cap needs to be removed from the pH electrode first and set aside for use when storing the electrode long-term. This soaking cap contains pH 4 buffer and some potassium chloride (KCl).
Fill the membrane module with 15 to 20 drops of fill solution carefully and tap the membrane module to ensure there are no bubbles present behind the membrane.
Slide the membrane module over the pH electrode until the top of the module and the top of the electrode body line up.
Then fit the cap over the top of the module and screw it tight. Be careful to not rupture the membrane.
The assembled ammonia ISE needs to be conditioned by soaking in a standard solution for 15 minutes prior to use. When completing a two-point calibration (e.g. 1 and 10mg/L) use the low standard solution for the soaking.
Note: It is not required but it is best to allow the inner pH electrode to condition within the filled membrane module for at least 2 hours prior to use. This means that the assembled electrode can be placed in a low to mid-range standard during this time.
Calibrating the Ammonia ISE
Determination of effective ion concentration with ion selective electrodes is very technique sensitive. A great deal of care must be taken when you are calibrating your ammonia ISE so that you can obtain accurate and repeatable results.
Step 1: Connect the ammonia ISE
Connect the ammonia electrode to a meter that has a BNC input and can directly display ion concentration (e.g. display in mg/L), such as the YSI MultiLab 4010-2, or the MultiLab 4010-3. Ensure the instrument has been set-up to measure ammonia.
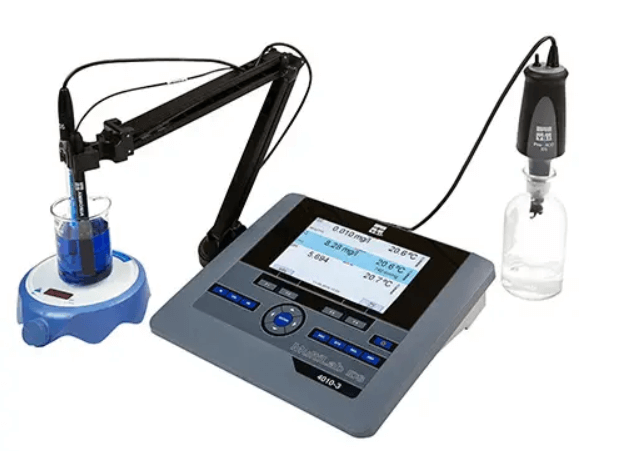
Step #2: Connect a temperature sensor
A temperature sensor then needs to be connected to the instrument, measuring effective ion concentration is dependent upon temperature. The standard solutions should have a temperature as close as possible to the expected sample.

Step #3: Prepare standards
Standards should bracket the expected sample range. There should be a tenfold difference in concentration between the high and low standards. Make sure standards are in date and use a minimum of two standards. You can also use a third standard if there is more than a tenfold difference.
Standards should be fresh and prepared very carefully. It is best to use a pipette when measuring small volumes of stock when preparing diluted standards.
Step #4: Place electrode in solution and stir
Place the electrode in the lowest concentration standard and stir at a constant rate using a stir bar and stir plate. The stirring speed should be limited to minimize loss of ammonia gas. Use the same stirring rate when calibrating and measuring samples.
Step #5: Add ISA and begin calibration

Add your ionic strength adjustor (ISA) to the standard and start calibration. Once the ISA is added, the solution should have a pale blue colour, indicating the pH >11 (gaseous ammonia). Allow the solution to stir for 1 minute and begin calibration. This is a delicate and time sensitive process. Ammonia in the sample will no longer be sufficient ~4 minutes after ISA is added, so after this time, a fresh standard will need to be prepared.
Step #6: Calibrate with additional standards
Once the instrument has accepted the first calibration point, finish calibrating using steps #4 and #5 for the remaining calibration points. Make sure to calibrate in order of increasing concentration. If calibrating with less than 7 standards, you can finish calibration process after calibration using the highest concentration standard.
Step #7: Evaluate electrode slope
For highest accuracy, the ammonia electrode slope should be between -53 mV/decade and -65 mV/decade. If not, recalibration is required. If the electrode slope is out of this range, attempt to recalibrate. Ensure your standards have carefully been prepared, ISA was used, and calibration to each point was completed 1 to 4 minutes after ISA was added.
If the electrode will not calibrate, you have ensured the electrode is correctly assembled, and you are confident your procedure is correct, attempt to clean the pH electrode and/or change the membrane module.
Step #8: Recalibration is Key
The ammonia electrode should be calibrated at the beginning of each day. Verify the calibration result every 2 hours by preparing a fresh low to mid-range standard, adding ISA, and verifying the reading. If the mV reading has changed ~3 mV compared to the reading in that standard during calibration, you will need to recalibrate the electrode.
Taking a Measurement
It is vital when samples are being prepared that they are using the same procedure used for standards. Steps 3 to 5 of the calibration procedure should be followed when preparing samples and taking a measurement.
In summary, 100 mL of each sample should be collected, and 2 mL of ISA should be added. The sample needs to be fresh and the same stirring rate used during calibration should be utilized. A measurement can be determined 1 to 4 minutes after ISA is added. The Auto-Read function of the TruLab and MultiLab can be used to ensure the measurement is stable.
Troubleshooting
If you are experiencing issues with calibration or poor response with the ammonia ion selective electrode, the following tips may be useful:
Refresh the internal fill solution
If the internal fill solution does not shift back to its original pH before another sample is taken, the next pH shift is not completely attributed to the new sample or standard. If you are having issues with poor slope or drifting readings, the electrode fill solution may need to be refreshed.
To ensure the internal fill solution pH shifts back to its original pH, you may need to:
- Refresh the fill solution manually so it is between the tip of the pH electrode and the membrane. This can be done by holding the electrode body sensor side down, pulling the cable back and away from the body/membrane slightly (it is spring loaded to cause the glass to seat up to the inside of the membrane) and then slowly releasing the cable. This pulls the glass away from the membrane, causing the internal fill solution to refresh quickly in the interface area
- Allow more time between measurements for the pH of the internal fill solution to return to normal
- Shaking out the old and refilling the membrane with new internal fill solution every few days might also be useful

Check for a tear in the membrane
When checking for a tear in the membrane, place the fully assembled electrode in pH 4 buffer. Since the membrane is only gas permeable, there should be no aqueous ion transfer between the inside and outside of the membrane. If the mV reading on the instrument display changes drastically once the electrode is placed in pH 4 buffer, there is a potential leak or tear in the membrane module.
Maintenance
If there is a poor response from the electrode, it may mean that the pH sensor might need to be cleaned so avoid rubbing or scratching the glass sensor when doing this.
It could be useful to condition the pH electrode by placing it in a pH 4 buffer along with some KCI. Ensure that the reference is completely immersed in the buffer solution.
The membrane module should be changed if it is dirty or stretched. Modules can last a long time in clean samples but dirty samples like wastewater where NH3 measurements are common, will have a shorter life.
Membrane modules need to be replaced when the electrode no longer calibrates or responds. Pinholes or tears will eventually develop from mashing the membrane into solids at the bottom of a beaker, get stretched, or the membrane will become fouled (surface coating).
You may attempt to clean a membrane module that has surface contamination by soaking it in DI water for 1 hour or more, then soak in a low or mid-range standard for at least 15 minutes prior to use.
Electrode Storage
Properly storing the ammonia ion selective electrodes will help ensure good electrode response and repeatable results.
Short-term storage
Between measurements, the ammonia ISE should be stored in a low concentration standard, such as 1 or 10 mg/L, with ISA added. For overnight or weekend storage, place the assembled electrode in 1000 mg/L standard and do not add any ISA. These short-term storage conditions will keep the assembled electrode stable for a comparably long period of time. Do not remove the membrane module for short term storage, as this can stretch the membrane, ultimately causing the electrode to be less responsive.
Long-term storage
Disassemble the electrode and rinse the module and pH electrode with DI solution then make sure all parts of the electrode are carefully dried. Place the pH electrode inside the included soaker bottle with pH 4 buffer and KCl added as a storage solution. The membrane module can be stored dry.
Common Questions - FAQs
Question: My electrode from another manufacturer was shipped dry. Is this OK?
Answer: It is recommended that no pH electrode is shipped dry. The storage solution the TruLine Ammonia ISE is shipped in contains the proper balance of moisture and salts to prevent leaching of reference electrolyte and the active ingredients of the glass membrane. If you are storing the electrode long-term, be sure to place the pH electrode inside the included soaker bottle with pH 4 buffer and KCl added as a storage solution. If you do not have KCl, pH 4 buffer will suffice.
Question: Do I need to recalibrate after using the spring-loaded cable to refresh the fill solution?
Answer: The internal fill solution (IFS) is homogenous, but an air bubble or other issues may change the tiny volume between the membrane and the tip of the pH electrode (i.e. the interface where the pH shift occurs). If the user is very consistent in their preparation (e.g. consistent number of IFS in the interface) and use of the electrode, recalibration is not needed. However, we recommend recalibration if the user is relatively new to the ammonia ISE.
Question: If measuring at low concentrations, is it OK to wait longer than 4 minutes after adding ISA to calibrate or measure?
Answer: It is not possible to generate more ammonia gas than what is in solution, so there is no reason to wait longer. Ammonia will still become gaseous at the same rate regardless of concentration. If the user waits longer than 4 minutes, ammonia will start to be depleted and the calibration/measurement result will be negatively impacted.
If you are measuring in low level samples and having difficulty, consider using multiple samples in low level measurement applications for highest accuracy. In addition, some customers have had success using Standard Method 4500-NH3 E (i.e. the known addition method) rather than Standard Method 4500-NH3 D (i.e. the direct calibration method) when calibrating.
They key to success with any gas-sensing ammonia electrode is to have a good, consistent technique!
Question: Is it best to insert the ammonia ISE at an angle so air bubbles don’t get trapped on the membrane?
Answer: The consistent presence of a bubble on the surface of the membrane is not a concern, as ammonia gas can easily permeate the bubble and then the membrane. In addition, inserting the ammonia ISE into the solution at an angle is not feasible on many electrode holders.
Although the consistent presence of a bubble is not a concern, bubbles sporadically moving across the membrane should be prevented. This sporadic movement of bubbles across the surface of the membrane should not occur if the membrane is all the way in solution and the stir plate speed is not turned up too high.
Question: How often should I replace the membrane and/or the internal fill solution (IFS)?
Answer: There is no general guideline for this, as the replacement interval simply depends how often the ammonia ISE is used, the samples measured (are they dirty?!), and the storage practices used. Some customers that use the ammonia ISE every day choose to replace the IFS once a week and the membrane once a month, while other customers only change the membrane and IFS when needed.
If experiencing issues, we recommend recalibrating first. If that does not resolve the issue, change the IFS. If issues persist, change the membrane. The internal pH electrode should be the last component replaced.
Indications that a membrane or IFS change is needed include a slow electrode response, the calibration slope is outside the recommended range, and/or the cable pull technique no longer helps.
Question: How often should I replace the internal electrode?
Answer: The internal pH electrode is just like any other pH electrode, so you should expect a similar usable life. Also, like other pH electrodes, proper storage of the pH electrode is critical! A pH electrode should never be stored dry or in deionized (DI) water.
Customers that use the electrode extensively should not have to replace their electrode any more than once a year. Some customers report getting at least two years of life out of their electrode. To maintain consistency, some customers choose to replace their electrode once a year.
Want to find out more about Ammonia Ion Selective Electrodes?
If you wish to find out more about ammonia ion selective electrodes calibration and measurements, contact a member of our team today and we will be happy to help!
Contact us Today! |
