Product Categories
Solid Phase Catalysis in Continuous Flow Chemistry
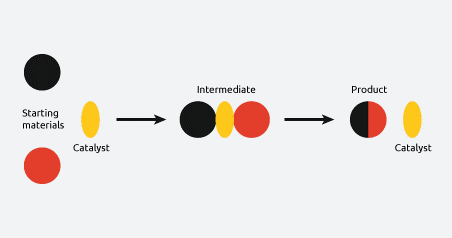
What is the meaning of catalysis?
Catalysis is the use of a catalyst to accelerate a chemical reaction. A catalyst is a substance that increases the rate of a chemical reaction without itself being used up in the reaction, enabling it to be recycled during the process. In continuous flow chemistry, a catalyst is often inserted in a packed bed reactor, and the reaction mixture is pushed thought the reactor using suitable pumping systems. By creating a different reaction pathway with lower activation energy, the catalyst reduces the amount of energy required by facilitating the process to get from point A to B, enabling a much faster reaction.
How does catalysis work?
Consider the reaction:
X + Y → Z (equation 1)
This reaction has a high activation energy (Ea) with no catalyst. When we introduce a catalyst, we provide an alternative pathway for the reaction to occur:
X + Y → X-Catalyst-Y → Z + Catalyst (equation 2)
In this example, a catalyst activates the reaction, resulting in the formation of an intermediate. The energy for the formation of the intermediate from the starting materials and the formation of the product from the intermediate has lower activation energy.
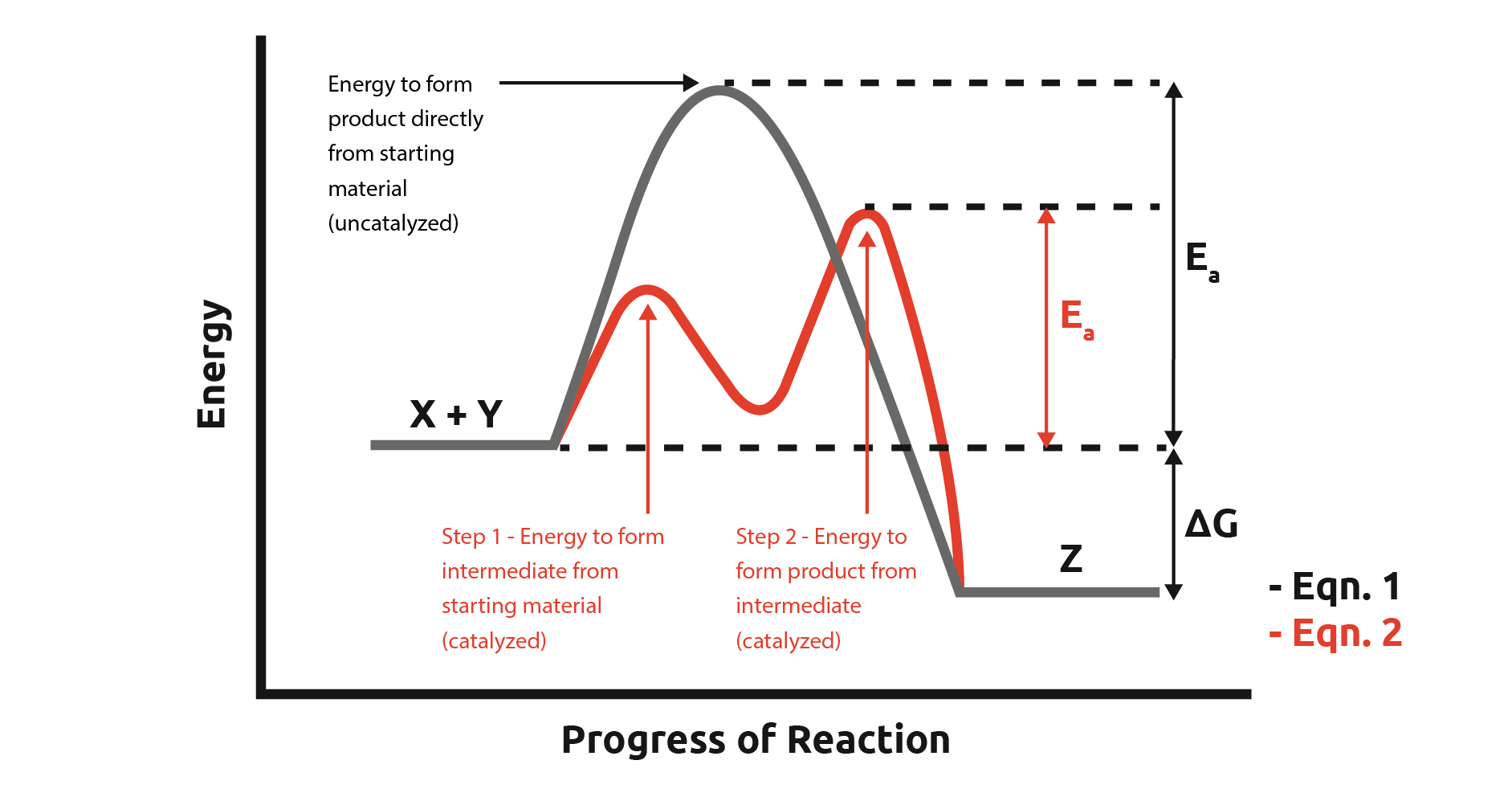
This activation typically occurs through the creation of an intermediate, or a series of intermediates. We can also see that our catalyst is regenerated at the end of the reaction and can be reused in subsequent reactions.
Note: The overall Activation Energy (Ea) of the reaction is the energy required to overcome the biggest step
Why would you want to incorporate a catalyst into your chemistry?
Catalysts enables much more efficient reactions:
- When we want to speed up our reaction rate
- By recycling the catalyst, we can increase the reaction yield in the same period of time as an uncatalyzed reaction.
- As the activation energy is lower, lower temperatures and environments can be used
- Catalysis can also increase the selectivity of certain reactions
What types of catalysis can you do in flow chemistry?

Homogeneous catalysis
Homogeneous catalysis occurs when the catalyst and the starting materials are in the same phase (gaseous, liquid, solid). This usually applies to catalysis that takes place in solution, with the catalyst and starting materials being miscible.
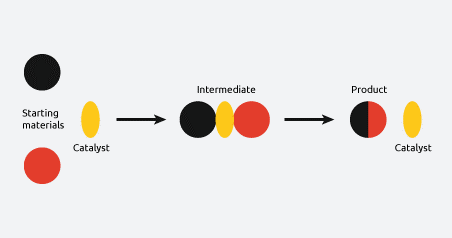
Heterogeneous catalysis
Heterogeneous catalysis refers to catalysis which occurs at the interface of two phases. This usually applies to gas-solid or liquid-solid reactions, with either phase serving as the initiator or the catalyst. These are solid surfaces, usually in the form of fine particles.
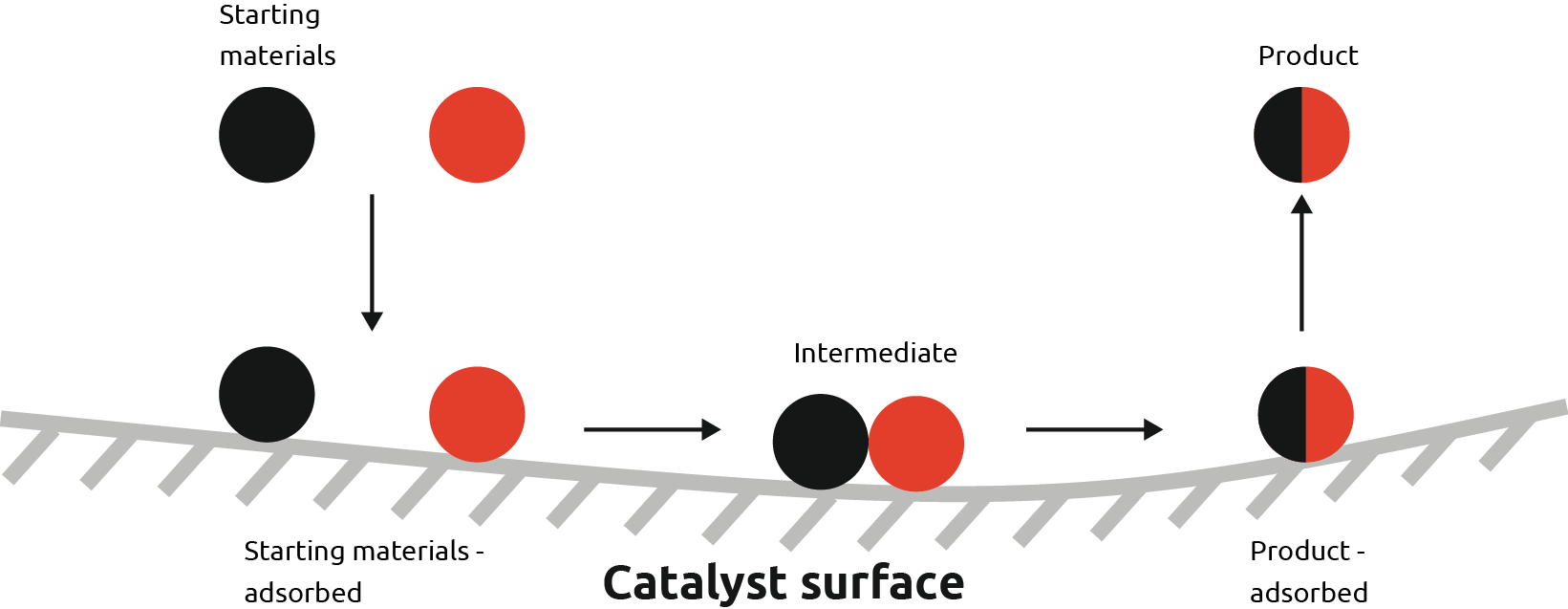
In these examples, the starting materials are adsorbed onto the surface where the intermediate is formed.

In flow chemistry, packed bed reactors are a popular way to perform heterogeneous catalysis, where you would fill a column with particles of a solid catalyst such as packing sand in a bucket on the beach and then flow through your reagents. The size of the particles can be altered depending on the chemistry required.
The nature of flow chemistry means you can alter the residence time and, as a result, the length of time your reagents are exposed to the catalyst surface.
Since catalysts are recycled and not used up, they can be left running for hours, allowing for a high production throughput.
Before adopting Catalysis in Continuous Flow Chemistry, check out our articles about 7 things to keep in mind when adopting flow chemistry and 5 Benefits of Automated Chemistry Systems.
Want to know more?
If you’re considering implementing continuous flow techniques into your lab, or even if you’re already working in flow but looking to improve your results, contact our Sales Team.
Contact us Today! |
